Abstract
Introduction: Treatment for acute lymphoblastic leukemia (ALL) with asparaginase (ASP) and glucocorticoids, such as dexamethasone (DEX), can cause changes in serum triglycerides (TGs). Glucocorticoids increase the activity of lipoprotein lipase (LPL), the enzyme responsible for removing TGs from the plasma, and increase production of TG-rich particles, resulting in the mobilization and redistribution of body fat. In contrast, ASP decreases the activity of LPL. Therefore, when glucocorticoids and ASP are given together, it is likely that TG-rich particles are rapidly formed, but insufficiently cleared, resulting in elevated serum TGs. The ASP formulation, e.g., native E. coli ASP (Elspar, L-ASP) vs. PEGylated ASP (Oncaspar; PEG-ASP), may influence the magnitude of TG changes. Our aim was to compare changes in TGs, the incidence of hypertriglyceridemia (hyperTG), and associated toxicities in two front-line pediatric St. Jude ALL trials that used different formulations of ASP: Total XV (L-ASP) and Total XVI (PEG-ASP).
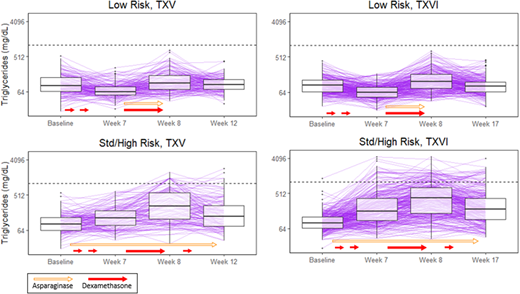
Methods: Patients on TXV (n=392) and TXVI (n=583) were assigned to the low-risk (LR) or standard/high risk (SHR) therapeutic arms. Serum TGs were measured as research biomarkers at four time-points: consolidation day 15 ("baseline" was > 35 days after the last dose of ASP during remission induction and > 21 days after the last dose of glucocorticoid); day 1 of week 7, 8, and either week 12 (TXV) or week 17 (TXVI) of continuation. Patients received DEX for 5 days/week during weeks 1, 4, and 14, for 8 days at week 7, and for 7 days at week 9 of continuation. In TXV, SHR patients received L-ASP weekly during the observed course of continuation (25,000 U/m2/dose); LR patients received L-ASP thrice weekly during continuation weeks 7-9 (10,000 U/m2/dose). In TXVI, patients were randomized to receive PEG-ASP 2,500 or 3,500 U/m2/dose during continuation: SHR weeks 1, 3, 5, 7, 9, 11, 13, and 15; LR weeks 7 and 9. HyperTG was defined as TG > 1000 mg/dL (dotted horizontal line in Figure 1). Clinically relevant toxicities in TXV, including thrombosis (≥ grade 3), osteonecrosis (≥ grade 2) and pancreatitis (≥ grade 3) were graded according to CTCAE v2.0; in TXVI CTCAE v3.0 was used, with identical grades except for pancreatitis (≥ grade 2).
Results: Similar trends in TGs were observed in both protocols for LR patients: there was a decrease in TGs from baseline to week 7, when DEX but not ASP had been given (TXV: p= 2 x 10-8, TXVI: p< 2 x 10-16); TGs increased from week 7 to 8 after both DEX and ASP (TXV: p=3 x 10-14, TXVI: p< 2 x 10-16); and the week 12/17 TG did not differ from baseline (> 16 days after last dose of DEX and ASP, TXV: p= 0.7, TXVI: p= 0.1). Among LR patients, the only difference between protocols was that the week 12/17 TG was higher on TXVI (with PEG-ASP) than TXV (with L-ASP) (p= 0.04). In contrast, among SHR patients, TGs were elevated at all time points relative to baseline (e.g. week 7 compared to baseline, TXV: p= 1 x 10-5, TXVI: p < 2 x 10-16); TGs increased from week 7 to 8 after both DEX and ASP (TXV: p= 6 x 10-16, TXVI: p < 2 x 10-16); and the week 12/17 TG remained higher than baseline (TXV: p= 9 x 10-9, TXVI: p < 2 x 10-16) at 7-16 days after last dose of DEX or ASP. Among SHR patients, TGs were higher in TXVI vs. TXV after baseline: at week 7 (p= 8 x 10-7), week 8 (p= 6 x 10-4), and at the week 12/17 TG (p= 8 x 10-4), suggesting a greater effect of PEG-ASP vs. L-ASP in increasing TGs. More patients developed hyperTG on TXVI vs TXV: 10% (59/583) vs. 5% (20/392), respectively (p= 0.007). In both protocols, only patients on SHR therapy developed hyperTG, suggesting an association with ASP. Patients with hyperTG were more likely to be diagnosed with thrombosis and osteonecrosis. However, this association was only significant after adjustment for age and risk group in TXVI: 17% of patients with vs. 4% without hyperTG developed post-induction thrombosis (p= 0.009); 39% of patients with vs. 11% without hyperTG developed osteonecrosis (p= 0.006). In TXV, associations of hyperTG with thrombosis (p= 0.2) and osteonecrosis (p= 0.08) were not significant after adjustment for age and risk group. There was no association between hyperTG and pancreatitis in either protocol.
Conclusions: These data support a greater association of PEG-ASP than L-ASP with elevation of serum TGs, and a greater effect of ASP than DEX. Whether serum hyperTG contributes to toxicities such as thrombosis or osteonecrosis, or is a biomarker of greater drug exposure or sensitivity, is unclear.
Inaba:Shire: Research Funding. Relling:Shire Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal